How to guide: Contracts
Some form of written agreement should be in place before a practice can start a study. Before agreement, the practice can discuss and plan for its involvement, but not recruit or start communicating with patients about the study.
Key information you should be aware of:
Agreements are put in place for any research or patient identification activity – this protects the practice as it clarifies what the practice is undertaking and makes sure all parties understand.
The Health Research Authority (HRA) strongly encourages the use of the Organisation Information Document (OID) or model agreements for agreement between studies and sites. When using a model agreement, the legal review has already been undertaken, so sites do not need to seek separate legal advice.
CTIMP or clinical device studies must have a written agreement – this is a legal requirement under clinical trials regulations.
Participant Identification Centre (PIC) sites should use the PIC agreement (model PIC agreement recommended). Studies can use other forms of agreement but practices may wish to seek legal advice if studies ask to use another agreement, as other agreements have not been verified by the HRA.
Note that a ‘signed’ agreement (even if not signed with a wet ink signature) is legally binding.
If practices are not sure of the requirements, they can contact West Yorkshire Research and Development (research@bradford.nhs.uk). WY R&D also offers advice to researchers, and can liaise with researchers about working with sites.
-
Signing up to be involved in a research study may involve signing a legally binding contract.
-
The HRA recommends that studies use model agreements which are standard templates having undergone review and confirmed as appropriate.
-
The agreement can only be signed by a person authorized by the practice to do this on the practice’s behalf.
-
Some studies will accept an email as confirmation of participation. Again, this should only be undertaken by an authorized person.
-
Some of these contracts take an ‘informal’ form but are still binding.
-
Studies may use other types of agreement but in these cases, practices may wish to seek legal advice.
-
The decision to participate, and the agreement, should be at the level of the participating organisation, i.e. usually at the level of the practice (as an independent contractor). PCNs are not able to sign a contract unless they have been set up as a company, and neither a PCN nor a federation is not able to sign a contract on behalf of its members unless there is a formal agreement that they can do this. If the research is taking place at the federation level, e.g. in the out of hours service, then this could be signed off by the federation. The ICB cannot sign off on behalf of practices.
See also
-
Getting your practice ready to do research guide
-
Roles and terminology guide
-
Principal Investigator guide
-
Data protection guide
Full guide
A contract will define roles and expectations, and in some cases is a legal requirement before a study can go ahead. It is helpful to ensure everyone has the same understanding of what is happening and who will carry out each activity, especially where transfers of data or tissue are involved, or financial arrangements.
Activity which can happen before a contract is signed:
Pre- agreement work can include:
-
Feeding into the planning and development of a study
-
Searching the practice database to see whether there are enough patients (to give an indicative number to the study team, but not contacting patients or sending any patient identifiable data to the study team)
-
Feasibility assessment to determine whether the practice can take part
-
Discussion with the study team and receiving documents to review the study
-
Signing of a non-disclosure agreement (sometimes required by commercial studies)
Studies not requiring a contract:
There will usually be no contract needed for studies which don’t require formal confirmation of capability and capacity. Practices are encouraged to confirm their participation via email to the study team. This would also enable the study to start as soon as the email (sent from an authorised member of staff from the practice) is sent.
Read more here: https://s3.eu-west-2.amazonaws.com/www.hra.nhs.uk/media/documents/collaborative-working-with-NHS-organisations-version-2-2-03-2018.pdf
If undertaking a study where the only activity is putting up a poster in the practice, then a contract will not normally be required.
Projects considered as service evaluation, rather than research, will not necessarily require a contract to be in place, but a data sharing or data processing agreement may be required if any data is involved. Contact us (research@bradford.nhs.uk) if you are in any doubt.
Who should sign?
The agreement should be signed by a person empowered to agree to the commencement of the research.
The practice should agree who will take on this role.
As the legal owners of the practice, the GP partners will usually be the people determining who is empowered to sign the agreement.
The ICB cannot sign any documents on behalf of the practice.
The decision to participate, and the agreement should be at the level of the participating organisation, i.e. usually at the level of the practice, unless the PCN or federation is the participating organisation, in which case the decision and agreement can be made by the PCN or federation. There must be a contractual relationship between the PCN or federation and the practice to allow the federation to conduct research on behalf of the practice. Contracts must be signed by an appropriate person with delegated authority by the practice.
What should happen before we sign?
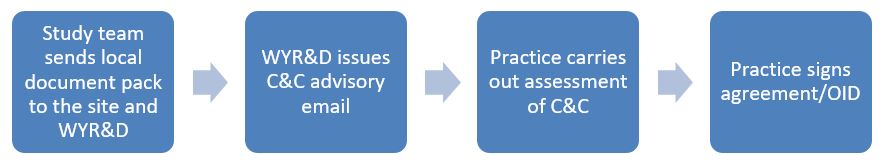
Before signing, the practice should have completed an assessment of capacity and capability (C&C) to participate (see the guide above to assessing, arranging and confirming capacity and capability). The sponsor/study team should have sent all applicable documents to the practice, as well as sharing a copy with the local R&D team (West Yorkshire R&D). Practices should direct study teams to contact WY R&D (research@bradford.nhs.uk) and send the local documents, if not done already.
As your R&D department, the West Yorkshire R&D team will assess whether there are any potential issues in delivering the study, and will issue a C&C Advisory email to provide any advice we think will help practices in getting set up. WY R&D will send this to study teams, who can share this with practices to advise them. Practices can also contact WY R&D with any questions about the study or about their C&C assessment. See also the guide for assessing, arranging and confirming capability and capacity.
Types of agreement:
Below is a guide to the types of agreement usually used in research.
Organisation Information Document:
The Organisation Information Document (OID) is usually used to put in place arrangements between practices and the study (for non-commercial studies). It acts as a formal written agreement, including finances, transfer of biological samples, data sharing, data processing and intellectual property.
Appendix 1 of the OID confirms whether the sponsor intends to use the OID as the agreement with sites. Note that the OID becomes a legal contract between the practice and the sponsor. The appendices are part of the legal contract, if indicated within each appendix.
Signing of the OID counts as a practice’s confirmation of capacity and capability. The outline OID is the approved HRA document; following approval of the study the exact content of the OID is to be negotiated and agreed between the practice and the sponsor – for example the local PI, and the number of participants to be recruited. The OID is then revised as the ‘localised organisation information document’ and this is the one which should be signed.
If you would like to read more, the guidance for the OID can be found here: https://www.myresearchproject.org.uk/help/help%20documents/Guidance_Organisation_Information_Document_v1-8_April_2024.pdf
Signing the OID/contract:
It is not intended that wet ink signatures are used, so completing the authorisation section and returning the document by email (from an authorised person), counts as a signature.
Organisation information document for data processing:
If only undertaking data processing (for a non-commercial study), the recommended contract is a simplified Organisation Information Document (see this template).
Model agreements:
For Clinical Trials of Investigational Medicinal Products (CTIMPs) and Clinical Trials of Medical Devices, a written agreement is a legal requirement. The recommended agreement is the model agreement (unmodified).
Model agreements are available for research and for PIC activity. The HRA recommends that unmodified model agreements are used. You can look at the model agreements here.
Where other agreements or modified model agreements are used, practices may wish to seek advice.
PIC agreements:
A PIC agreement should be in place when the practice acts as a PIC for a study. See the guide on PICs for further information about being a PIC.
Use of an unmodified model PIC agreement is recommended. The agreement is put in place between the PIC and the research site – so for example a local Trust.
Other contracts:
If there are any Service Support Costs to be paid by the Research Delivery Network (RDN), practices will need to have a contract in place with the RDN (which puts in place payment arrangements). If you do not have a person to contact from the RDN, please contact research@bradford.nhs.uk and we will put you in touch.
Practices may want to consider implementing confidentiality agreements for researchers wanting to access their practice, as an extra reassurance. There is a template on the IRAS website: link
You may, usually for commercial studies, be asked to sign a non-disclosure agreement, which is used to ensure that commercially sensitive information is protected. This is common practice, but practices should ensure that they are happy with the terms and ensure that they can keep to the requirements. Be aware that these are legally binding so practices should be aware of their responsibilities under the contract and ensure they keep the confidential documents to the confidentiality standard required.
Glossary of Acronyms and Terms:
-
CRN - Clinical Research Network
-
GCP - Good Clinical Practice training
-
OID - Organisation Information Document
-
PI - Principal Investigator
-
PIC - Participant Identification Centre
-
SIV - Site Initiation Visit
-
SOPs - Standard Operating Procedures
-
CTIMP - Clinical Trial of an Investigational Medicinal Products
-
PCN - Primary Care Network