

Research can take many forms and there are lots of different ways you could take part. You might be asked to help to investigate a new drug or treatment, to help look at an existing treatment in a new way, to complete a questionnaire or to take part in an interview to collect information about how you feel about an NHS service or your experience of treatment. A lot of people like taking part in health research and value the contribution they can make to medical science.
As well as being a participant in a research study you can also get involved in other ways:
-
Consulting on studies - It is important that researchers do research with patients, rather than 'to', 'for' or 'about' them.
-
Reading and sharing findings - It's also really important that findings of a research study are made available, enabling patients to be able to see when research has led to an outcome which could help the NHS to utilise the best, most cost-effective treatments. All clinical trials which take place in the EU (these are research studies testing a treatment) have to be registered so that information about them is publicly available. Anybody can access information about the study (although they are not allowed to access information about patients taking part or any personal data).
-
Getting involved - If you have an idea that you think should be the focus of a research study, or that you think should be highlighted to researchers as an area they could look at, you could feed this into possible future studies.
The NIHR Yorkshire and Humber Research Delivery Network (RDN) funds health and care research, translating discoveries into practice. The NIHR’s vision is to improve the health and wealth of the nation through research, involving patients and the public in all of their work. There is a comprehensive section about patient and public involvement in research on the NIHR website: https://www.nihr.ac.uk/career-development/health-and-care-research-introduction/involve-patients
The NIHR Yorkshire and Humber Research Delivery Network (RDN) is a Regional Research Delivery Network (RRDN). There is a lot of support available through our local RRDN for patients and the public who would like to be involved in research.
The NIHR has also released a guide: Briefing Notes for Researchers - public involvement in NHS, health and social care research.
The NIHR Research Champion initiative is supported by NIHR Research Delivery Networks across the country. The NIHR advises that "Research Champions volunteer their time to help spread the word about health and care research to patients and the public, and especially those groups who are currently less likely to take part in research."
If you are enthusiastic about health research and would like more information about becoming a Research Champion in the Yorkshire and Humber area then you can contact the NIHR to discuss.
In this short video John White explains why he volunteers as a Research Champion (previously known as Patient Research Ambassadors):
For more information about what being a Research Champion involves please visit the NIHR website:
Be Part of Research exists to help you find out about health and social care research that is taking place across the UK.
Website: https://bepartofresearch.nihr.ac.uk/
Join Dementia Research is a national service that enables you to register your interest and be matched with suitable research studies. People with dementia or memory problems, their carers or anyone who is interested can sign up.
Website: https://www.joindementiaresearch.nihr.ac.uk/
EMRI is a network of research staff and members of the public from across the Yorkshire and Humber region with a shared interest in increasing levels of participation in health research within Ethnic Minority communities throughout Yorkshire and Humber.
Website: https://sites.google.com/view/emrinetwork/home
The James Lind Alliance facilitates groups where patients and clinicians can get together to identify areas which should be a priority for particular disease or topic areas. There are opportunities presented through the James Lind Alliance for you to have your say about your personal research priorities.
Website: https://www.jla.nihr.ac.uk/priority-setting-partnerships/
People in Research is a database of opportunities for members of the public who have an interest in getting involved in research. You can search opportunities for involvement in research. You can also sign up to receive email alerts if opportunities arise in an area of research that is of particular interest to you.
Website: www.peopleinresearch.org
It’s important that the findings of research studies are shared so that patients can make decisions with the backing of quality evidence. If you would like to read some of the findings of local research studies, please visit our repository.
Website: www.westyorksrd.nhs.uk/repository
The Research Engagement Network (REN) project:
REN was launched in West Yorkshire in November 2023 and is a voluntary, community and social enterprise sector (VCSE) led project, funded by NHS England, through which VCSE Research Champions have engaged with and delivered sessions with people from an identified underserved community:
-
to develop an understanding of research barriers and enablers and to explore opportunities for myth-busting around research;
-
to offer opportunities for research participation;
-
to understand the kinds of information communities are willing to share as part of research.
Further information and case studies will be available soon.
Kirklees VCSE research champions case study:
Rehana's case study:
West Yorkshire Research Engagement Network (REN) contributors:
With thanks to all research champions, communities and partners, including:
-
Bradford District & Craven: Black Health Forum (Bradford), Bangladeshi Youth Organisation, the Race Equality Network, the VCS Alliance;
-
Calderdale: Jah Light Community Project, Mums On A Mission, Calderdale Voluntary and Community (CVAC);
-
Kirklees: Community Skills Centre, Ravensthorpe Community Centre, Third Sector Leaders Kirklees;
-
Leeds: Hamara, Hope Bereavement Support, LeedsACTS!, Forum Central, Voluntary Action Leeds;
-
Wakefield: One Ummah, Spectrum People, Nova Wakefield, Wakefield Council;
-
everyone who took part in community conversations with the research champions;
-
the West Yorkshire Power of Communities (previously HPoC) programme;
-
the West Yorkshire VCSE Voices Panel;
-
the Ethnic Minority Research Inclusion (EMRI) network;
-
the Co-production and Peer Research (CoPPer) network;
-
the National Institute for Health and Care Research (NIHR) Clinical Research Network Yorkshire and Humber;
-
the West Yorkshire Integrated Care Board (ICB);
In the NHS, we are very careful to make sure that researchers who want to work with our patients are carrying out safe, ethical, well considered research. It is important to make sure that patients’ rights and well-being are considered, and that research is in their best interests.
We have to make sure that the benefit of the research outweighs any harm or inconvenience. If the research is not planned or thought-through properly, then it may be that the findings are not useable. This would mean that everyone involved will have wasted their time and money. Any study that comes to your GP or other NHS site will have been checked, and special checks are in place if the research involves radiation, genetic material, human tissues, children, embryos or prisoners.
Some studies are funded by drug companies or companies which manufacture medical devices. These go through the same checks as any other study, to make sure that the research is asking a valuable question and is properly conducted – not just in the interest of the company.
It is important for new drugs and devices to be tested, first and foremost so that we know if they are safe, but also so that we know if there are any issues with using them (for example side effects or if patients in a real-life setting find them too difficult or unpleasant to use). All of these trials are very strictly regulated. But they are also important as they are necessary for new treatments to be developed. A drug or device goes through a lot of checks and processes before it comes to patient testing.
If patients are asked to take part in a research study (and they should always be asked, not forced or coerced), they should be given information about the study (including any risks), should have the opportunity to ask any questions, and will give their consent to take part. For people who cannot consent (children, some vulnerable adults, people who are unconscious) there have to be special arrangements to make sure their rights are protected. If someone cannot give written consent (e.g. If they cannot read or write) there must be a suitable alternative. These are all things an ethics committee will look for when agreeing for a study to take place. This can also be checked at any time if a study is audited. A patient can change their mind and withdraw their consent if they want to.
These checks also make sure that the people who are undertaking the research are properly qualified and trained.
An easy read guide covering the topics discussed in this section is available here.
What questions do we ask?
-
Is it ethical?
-
Have patients had the opportunity to feed into the research – to make sure we are asking a relevant question and have considered any particular issues for the patient group being studied?
-
Do all relevant patients have the same opportunity to be involved? For example, does the research exclude a patient because they don’t speak English or can’t read?
-
Are the researchers studying a big enough patient group for the findings to be generalizable to others?
-
Are the researchers looking after the patients in the study – in particular looking after their health and well-being, looking after their data and giving them the opportunity to withdraw from the study if they want?
-
Might patients feel pressurised into taking part? The researchers should reassure the patients that they don’t have to take part and they won’t be disadvantaged if they don’t take part.
-
Have the researchers made proper arrangements for any data or samples to be transported securely?
-
Who will have access to any patient confidential data?
-
Have the researchers been realistic in how many patients they believe they will get to take part?
Before a study starts in the NHS, it will have been through a process:
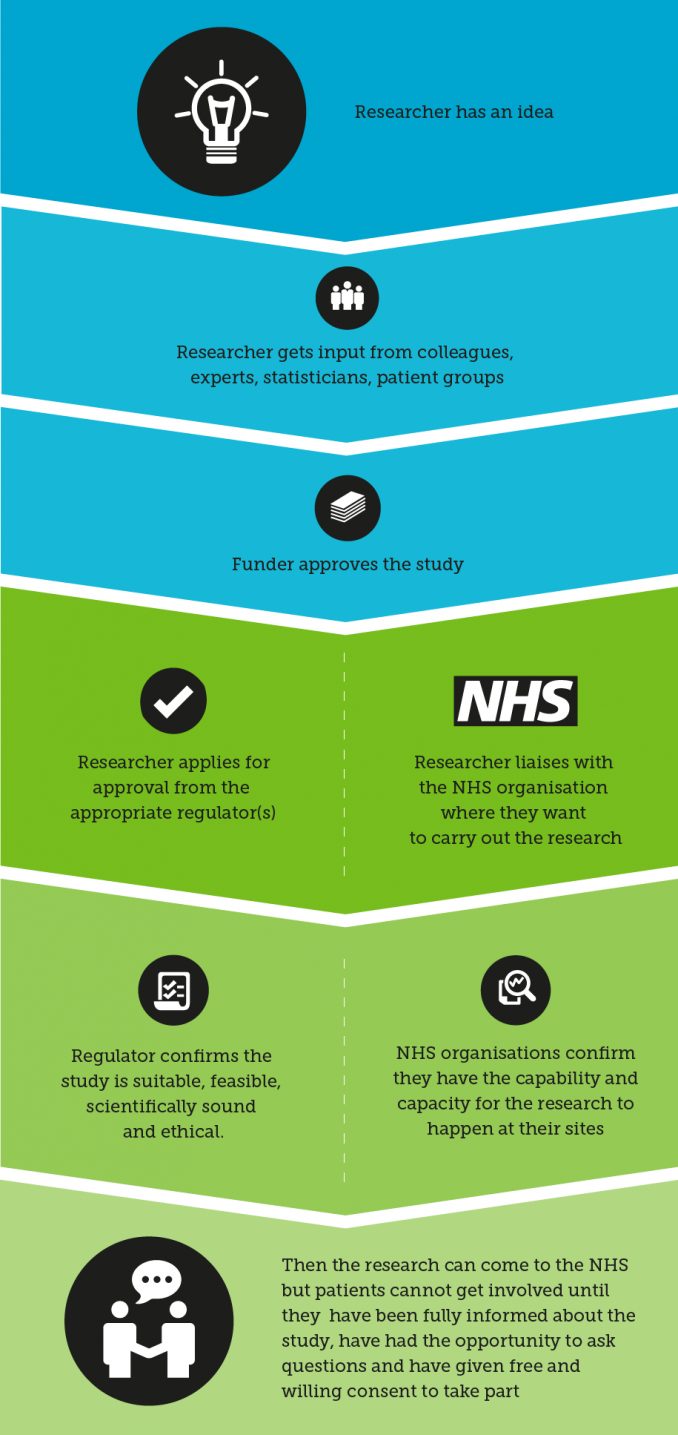
This process ensures the study has been checked by a number of sources before it takes place, and when a patient gets involved he or she can be confident that the study has considered all the relevant issues. If you are approached to get involved in research and are concerned about safety or any element of the study, there should always be a contact within the study who should be able to address any questions or concerns. You can also contact us if you are concerned about a study.
You can read more about how research is regulated here.
How do I know my data is safe?
You can read more about how your data is handled in research by visiting the Health Research Authority website:
https://www.hra.nhs.uk/information-about-patients/
You have the choice to decide whether your data can be used for research. We call this the ‘opt-out’ process. NHS Digital has a page dedicated to the national data opt-out and mythbusting:
https://digital.nhs.uk/services/national-data-opt-out/mythbusting-social-media-posts
To read more about what research is from the patient's perspective you can access a number of resources from the National Institute for Health and Care Research here:
https://www.nihr.ac.uk/career-development/health-and-care-research-introduction/involve-patients












