In the NHS, we are very careful to make sure that researchers who want to work with our patients are carrying out safe, ethical, well considered research. It is important to make sure that patients’ rights and well-being are considered, and that research is in their best interests.
We have to make sure that the benefit of the research outweighs any harm or inconvenience. If the research is not planned or thought-through properly, then it may be that the findings are not useable. This would mean that everyone involved will have wasted their time and money. Any study that comes to your GP or other NHS site will have been checked, and special checks are in place if the research involves radiation, genetic material, human tissues, children, embryos or prisoners.
Some studies are funded by drug companies or companies which manufacture medical devices. These go through the same checks as any other study, to make sure that the research is asking a valuable question and is properly conducted – not just in the interest of the company.
It is important for new drugs and devices to be tested, first and foremost so that we know if they are safe, but also so that we know if there are any issues with using them (for example side effects or if patients in a real-life setting find them too difficult or unpleasant to use). All of these trials are very strictly regulated. But they are also important as they are necessary for new treatments to be developed. A drug or device goes through a lot of checks and processes before it comes to patient testing.
If patients are asked to take part in a research study (and they should always be asked, not forced or coerced), they should be given information about the study (including any risks), should have the opportunity to ask any questions, and will give their consent to take part. For people who cannot consent (children, some vulnerable adults, people who are unconscious) there have to be special arrangements to make sure their rights are protected. If someone cannot give written consent (e.g. If they cannot read or write) there must be a suitable alternative. These are all things an ethics committee will look for when agreeing for a study to take place. This can also be checked at any time if a study is audited. A patient can change their mind and withdraw their consent if they want to.
These checks also make sure that the people who are undertaking the research are properly qualified and trained.
An easy read guide covering the topics discussed in this section is available here.
What questions do we ask?
-
Is it ethical?
-
Have patients had the opportunity to feed into the research – to make sure we are asking a relevant question and have considered any particular issues for the patient group being studied?
-
Do all relevant patients have the same opportunity to be involved? For example, does the research exclude a patient because they don’t speak English or can’t read?
-
Are the researchers studying a big enough patient group for the findings to be generalizable to others?
-
Are the researchers looking after the patients in the study – in particular looking after their health and well-being, looking after their data and giving them the opportunity to withdraw from the study if they want?
-
Might patients feel pressurised into taking part? The researchers should reassure the patients that they don’t have to take part and they won’t be disadvantaged if they don’t take part.
-
Have the researchers made proper arrangements for any data or samples to be transported securely?
-
Who will have access to any patient confidential data?
-
Have the researchers been realistic in how many patients they believe they will get to take part?
Before a study starts in the NHS, it will have been through a process:
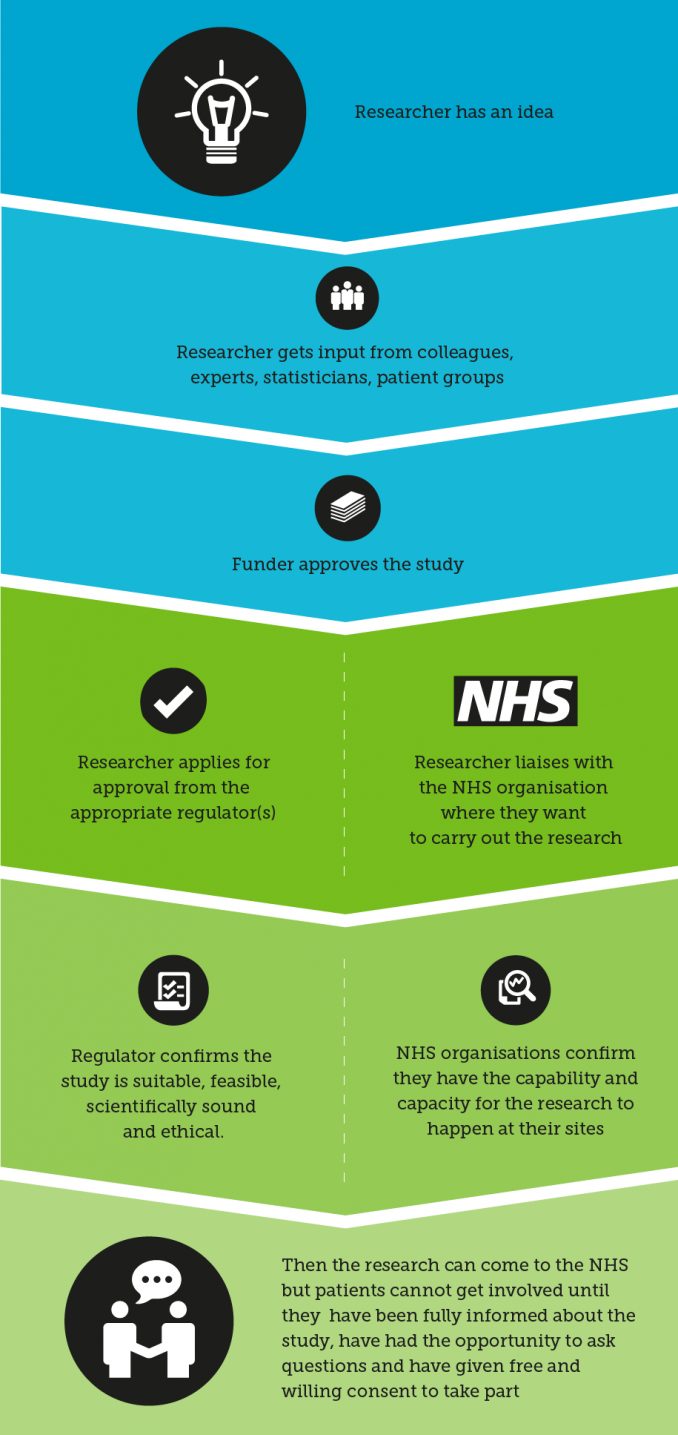
This process ensures the study has been checked by a number of sources before it takes place, and when a patient gets involved he or she can be confident that the study has considered all the relevant issues. If you are approached to get involved in research and are concerned about safety or any element of the study, there should always be a contact within the study who should be able to address any questions or concerns. You can also contact us if you are concerned about a study.
You can read more about how research is regulated here.
How do I know my data is safe?
You can read more about how your data is handled in research by visiting the Health Research Authority website:
https://www.hra.nhs.uk/information-about-patients/
You have the choice to decide whether your data can be used for research. We call this the ‘opt-out’ process. NHS Digital has a page dedicated to the national data opt-out and mythbusting:
https://digital.nhs.uk/services/national-data-opt-out/mythbusting-social-media-posts
To read more about what research is from the patient's perspective you can access a number of resources from the National Institute for Health and Care Research here:
https://www.nihr.ac.uk/career-development/health-and-care-research-introduction/involve-patients

