

The West Yorkshire R&D team helps to transform research questions into research proposals. The team has links with a diverse range of professionals, including clinicians and academics, and provides vast experience in research development. The team offers support in the following key areas:
-
Methodology
-
The feasibility of delivering a project within a NHS setting
-
Facilitation of key partnerships to promote successful delivery
-
Securing funding and project collaborators
West Yorkshire R&D has helped to establish a multi-million pound portfolio of research which informs decision-making by clinicians, patients and commissioners. Our research work is conducted so that health service staff are involved in the analysis and interpretation of emerging research findings as studies progress, enabling more timely and relevant research outputs. Examples of projects we have supported include: Action to Support Practices Implementing Research Evidence (ASPIRE); Understanding prescribing of opioids for chronic, non-cancer pain in general practice; Improving the Management of Pain from Advanced Cancer in the Community (IMPACCT); Self-Management of Analgesia and Related Treatments at the End of life (SMARTE); Electronic Palliative Care Co-ordination System (EPaCCS); and TIME4PallCare - Determining timely engagement with palliative care for patients with advanced cancer.
For more information or to discuss involvement in a research project please contact us.
You can read more about patient and public involvement in research through the public section of our website: www.westyorksrd.nhs.uk/public
We may sometimes collect information from researchers but always take care to treat it fairly. (How is my information used?)
Research governance can be defined as the broad range of regulations, principles and standards of good practice that exist to achieve and continuously improve research quality across all aspects of healthcare in the UK and worldwide. For the purposes of research governance, ‘research’ means the attempt to derive generalisable new knowledge by addressing clearly defined questions with systematic and rigorous methods.
Research governance applies to everyone connected to health and social care (this means any health-related research which involves humans, their tissue and/or data), whether as a chief/principal investigator, care professional, researcher, employer or as support staff. If you are involved in research of this kind it is important that you are aware of your obligations throughout the process. The UK Policy Framework for Health and Social Care Research sets out the responsibilities and standards that apply to work managed within the formal research context.
If you need help to decide whether your project is classified as research you can refer to the Health Research Authority decision tool.
Useful websites
Engagement in research is high on the political agenda. This is reflected in key Government publications including:
Through increased engagement in research we are able to enact the Government vision of promoting efficiency through innovation. Patient and public involvement in research is of paramount importance as it helps to demonstrate openness and enriches the research process through inclusion.
The West Yorkshire R&D team works closely with the National Institute for Health and Care Research (NIHR) Regional Research Delivery Network (RRDN) who are able to offer both practical and financial support for the delivery of research.
The team is currently working on several grant-funded projects with a number of organisations, including local universities.
The WY R&D team helps to ensure that commissioning decisions are based on robust evidence through the sharing of best practice. We communicate study findings and research opportunities, and we have hosted a number of events that provide a knowledge exchange forum for NHS healthcare professionals. We also support researchers to communicate and translate their findings into NHS settings, giving them opportunities to reach decision makers and practitioners.
We develop events where researchers can share evidence in areas of particular interest to commissioners and other health professionals in a timely manner to support their decision-making. Recent events have focused on cancer and palliative care, elderly care and frailty, mental health in primary care, earlier diagnosis of cancer and on improving prescribing.
The West Yorkshire R&D team seeks to share findings of research studies. Study teams looking to share their research findings can contact us to discuss ways of sharing research findings with GP practices, colleagues and commissioners. We have established a repository where end of study reports, research outputs or links to published articles can be shared and accessed.
To discuss possible topics for learning events, or to share your research findings, please get in touch.
Please see the 'How we can help' section below for practical ways in which we can help you access GP practices in an attempt to increase study recruitment, or read the below information and then do contact us if you've any further questions about finding sites and recruiting patients.
Read about: Events / Newsletters / Repository / Website
It is important that the findings of research are shared, as: healthcare practice moves forward through the use of research findings; commissioners use robust research evidence to support their commissioning decisions; research participants often value receiving the results of research they've been involved in as this recognises the importance of their contribution; other researchers can use research findings to inform their own research studies; and published research contributes to the growing body of knowledge which can be accessed by academics, clinicians, students, commissioners, or anyone with an interest in research. It is also important to share research findings even if nothing significant was discovered as this can prevent researchers from duplicating studies in the future.
Dissemination can take many forms. The most traditional is a published journal article. Journals will usually have a website which gives instructions on submitting. Many research funders require research findings to be published in an open access publication.
You can also contact the press with any key findings, in particular in ‘hot topic’ areas. For advice on writing a press release see here.
You may want to promote a finding or an article via social media, or via a research specific social media platform e.g. ResearchGate.
How we can help
We can help you to disseminate your research findings. Please contact our Research Engagement Officer if you would like to discuss research engagement and knowledge transfer opportunities in further detail. Some of these opportunities include:
You can read about our most recent events below:
If you would like to be informed about future events please contact us.
The West Yorkshire R&D Team feed into various newsletters, including those received directly by commissioners and health professionals. Please contact us if you would like to contribute information to these newsletters, for example if you need to: raise awareness of a research related event or training opportunity; increase awareness of and recruitment to a research study; disseminate results and learning from research; or generally raise awareness about research related activity. To disseminate information this way in the area please contact us to discuss.
The West Yorkshire R&D Team can publish or link to articles in our repository.
We can share information about your research findings via our website and Twitter.
Research can take many forms and there are lots of different ways you could take part. You might be asked to help to investigate a new drug or treatment, to help look at an existing treatment in a new way, to complete a questionnaire or to take part in an interview to collect information about how you feel about an NHS service or your experience of treatment. A lot of people like taking part in health research and value the contribution they can make to medical science.
As well as being a participant in a research study you can also get involved in other ways:
-
Consulting on studies - It is important that researchers do research with patients, rather than 'to', 'for' or 'about' them.
-
Reading and sharing findings - It's also really important that findings of a research study are made available, enabling patients to be able to see when research has led to an outcome which could help the NHS to utilise the best, most cost-effective treatments. All clinical trials which take place in the EU (these are research studies testing a treatment) have to be registered so that information about them is publicly available. Anybody can access information about the study (although they are not allowed to access information about patients taking part or any personal data).
-
Getting involved - If you have an idea that you think should be the focus of a research study, or that you think should be highlighted to researchers as an area they could look at, you could feed this into possible future studies.
The Research Engagement Network (REN) project:
REN was launched in West Yorkshire in November 2023 and is a voluntary, community and social enterprise sector (VCSE) led project, funded by NHS England, through which VCSE Research Champions have engaged with and delivered sessions with people from an identified underserved community:
-
to develop an understanding of research barriers and enablers and to explore opportunities for myth-busting around research;
-
to offer opportunities for research participation;
-
to understand the kinds of information communities are willing to share as part of research.
Further information and case studies will be available soon.
Kirklees VCSE research champions case study:
Rehana's case study:
West Yorkshire Research Engagement Network (REN) contributors:
With thanks to all research champions, communities and partners, including:
-
Bradford District & Craven: Black Health Forum (Bradford), Bangladeshi Youth Organisation, the Race Equality Network, the VCS Alliance;
-
Calderdale: Jah Light Community Project, Mums On A Mission, Calderdale Voluntary and Community (CVAC);
-
Kirklees: Community Skills Centre, Ravensthorpe Community Centre, Third Sector Leaders Kirklees;
-
Leeds: Hamara, Hope Bereavement Support, LeedsACTS!, Forum Central, Voluntary Action Leeds;
-
Wakefield: One Ummah, Spectrum People, Nova Wakefield, Wakefield Council;
-
everyone who took part in community conversations with the research champions;
-
the West Yorkshire Power of Communities (previously HPoC) programme;
-
the West Yorkshire VCSE Voices Panel;
-
the Ethnic Minority Research Inclusion (EMRI) network;
-
the Co-production and Peer Research (CoPPer) network;
-
the National Institute for Health and Care Research (NIHR) Clinical Research Network Yorkshire and Humber;
-
the West Yorkshire Integrated Care Board (ICB);
In the NHS, we are very careful to make sure that researchers who want to work with our patients are carrying out safe, ethical, well considered research. It is important to make sure that patients’ rights and well-being are considered, and that research is in their best interests.
We have to make sure that the benefit of the research outweighs any harm or inconvenience. If the research is not planned or thought-through properly, then it may be that the findings are not useable. This would mean that everyone involved will have wasted their time and money. Any study that comes to your GP or other NHS site will have been checked, and special checks are in place if the research involves radiation, genetic material, human tissues, children, embryos or prisoners.
Some studies are funded by drug companies or companies which manufacture medical devices. These go through the same checks as any other study, to make sure that the research is asking a valuable question and is properly conducted – not just in the interest of the company.
It is important for new drugs and devices to be tested, first and foremost so that we know if they are safe, but also so that we know if there are any issues with using them (for example side effects or if patients in a real-life setting find them too difficult or unpleasant to use). All of these trials are very strictly regulated. But they are also important as they are necessary for new treatments to be developed. A drug or device goes through a lot of checks and processes before it comes to patient testing.
If patients are asked to take part in a research study (and they should always be asked, not forced or coerced), they should be given information about the study (including any risks), should have the opportunity to ask any questions, and will give their consent to take part. For people who cannot consent (children, some vulnerable adults, people who are unconscious) there have to be special arrangements to make sure their rights are protected. If someone cannot give written consent (e.g. If they cannot read or write) there must be a suitable alternative. These are all things an ethics committee will look for when agreeing for a study to take place. This can also be checked at any time if a study is audited. A patient can change their mind and withdraw their consent if they want to.
These checks also make sure that the people who are undertaking the research are properly qualified and trained.
An easy read guide covering the topics discussed in this section is available here.
What questions do we ask?
-
Is it ethical?
-
Have patients had the opportunity to feed into the research – to make sure we are asking a relevant question and have considered any particular issues for the patient group being studied?
-
Do all relevant patients have the same opportunity to be involved? For example, does the research exclude a patient because they don’t speak English or can’t read?
-
Are the researchers studying a big enough patient group for the findings to be generalizable to others?
-
Are the researchers looking after the patients in the study – in particular looking after their health and well-being, looking after their data and giving them the opportunity to withdraw from the study if they want?
-
Might patients feel pressurised into taking part? The researchers should reassure the patients that they don’t have to take part and they won’t be disadvantaged if they don’t take part.
-
Have the researchers made proper arrangements for any data or samples to be transported securely?
-
Who will have access to any patient confidential data?
-
Have the researchers been realistic in how many patients they believe they will get to take part?
Before a study starts in the NHS, it will have been through a process:
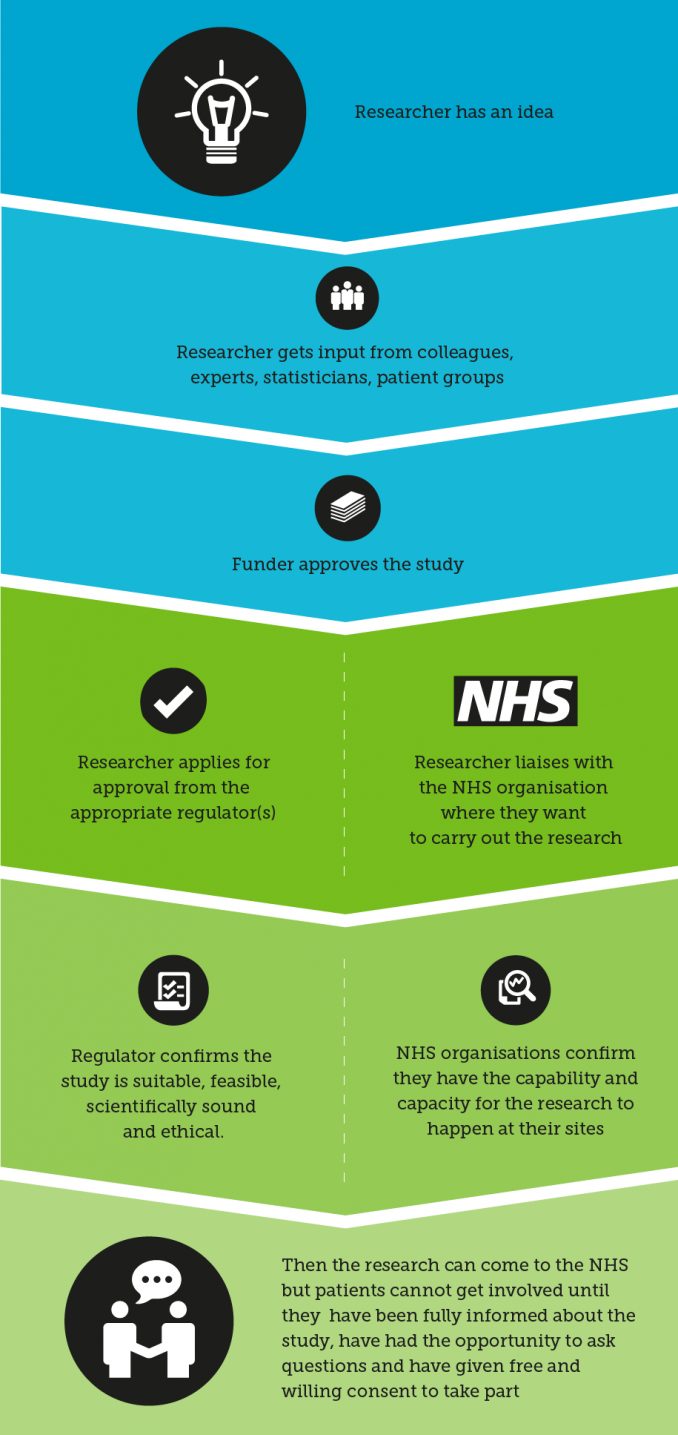
This process ensures the study has been checked by a number of sources before it takes place, and when a patient gets involved he or she can be confident that the study has considered all the relevant issues. If you are approached to get involved in research and are concerned about safety or any element of the study, there should always be a contact within the study who should be able to address any questions or concerns. You can also contact us if you are concerned about a study.
You can read more about how research is regulated here.
How do I know my data is safe?
You can read more about how your data is handled in research by visiting the Health Research Authority website:
https://www.hra.nhs.uk/information-about-patients/
You have the choice to decide whether your data can be used for research. We call this the ‘opt-out’ process. NHS Digital has a page dedicated to the national data opt-out and mythbusting:
https://digital.nhs.uk/services/national-data-opt-out/mythbusting-social-media-posts
To read more about what research is from the patient's perspective you can access a number of resources from the National Institute for Health and Care Research here:
https://www.nihr.ac.uk/career-development/health-and-care-research-introduction/involve-patients
RCGP has an initiative, working together with National Institute for Health and Care Research (NIHR) to identify areas for research. To contribute to this, and influence research from a primary care perspective, see this link.
If you are interested in developing your own study, please read here.
What do I need to know about the research governance process?
There are a number of ways you can get involved in research – each with different levels of involvement.
You may have seen developments in regard to OpenSAFELY, a new method for allowing anonymised aggregated data to be shared for research purposes. You should have received a letter which directed your practice to enable this in your practice system (SystmOne or EMIS/Optum).
We are happy to answer any questions you might have about OpenSAFELY, but have provided some information for your reference and to help you to address any queries from patients.
If you have been approached and are not sure whether you want to be involved in a study, or whether it has been through the appropriate checks, please see our guide below, or contact us for advice.
To find out about the study and exactly what activity the team would like to carry out, reading the protocol should give the essential information. This should tell you what exactly will be asked of you and your patients, so you can decide if you want to be involved. This is entirely voluntary. It could be as little as performing a database search or could be actually conducting research at your site.
Before a study can go ahead at a practice, there are a number of things you will need to see as a practice to make sure the researchers have gone through the proper checks. We have put together an outline checklist which may help you to know what documents you need to see.
As a starting point, you will probably want to think about:
-
Does the study interest us and have a benefit for our patient population?
-
Has the study been appropriately financed? i.e. will our time and facilities be financially covered?
-
What resources does the study require and do we have these available: e.g. Staff, skills, facilities?
-
How much time will the study take? For which staff members? Can we spare the capacity? Are the appropriate staff willing to be involved?
-
What arrangements would need to be put in place for the study to take place?
-
How many patients does the study team wish to recruit? Do we have sufficient patient population? Does our patient population have the demographics/conditions the study team is looking for?
-
Is there any special equipment required for the study? Do we have the required equipment?
-
Does the study require access to the clinical system – are they set up to use the clinical system that we use (some studies may only be able to work with SystmOne data, or EMIS data)?
-
If the study team is asking to access our facilities/patients/systems, do we accept this? Please note that they will need a Letter of Access issued by the ICB to access practices – this should be shown to the practice before they can access your site, and demonstrates that they have had pre-employment checks, DBS checks where appropriate and are suitably qualified.
-
Has the study team sufficiently considered how our patients’ data and/or samples will be handled and transported? Does this comply with our data protection requirements?
-
If there is a study treatment involved, does this cost more than standard treatment? If so, has the local clinical research network (CRN) agreed to pay for the excess? The study team should have a letter from the CRN stating that they agree to cover this cost.
-
Have there been any amendments to the study? Practices should make sure they review these as they can mean significant changes to what was originally proposed in the study documentation.
You may be asked to sign a contract, which will usually be a standard format (the National Institute for Health and Care Research advises that researchers use their standard template). Alternatively, the study team may want to use an organisation information document in place of a contract. If you are asked to sign any other contract you may wish to ask for legal advice.
Maybe this has piqued your interest, but you have decided that you don’t want to be involved with the particular study which approached you. To find out about other research in the area you can email us, or read more about developing your own study idea.
What next?
After reviewing the content of the study and gaining an understanding of what your practice is being asked to do, there are a few things you need to check before a study can go ahead.
There are extensive checks carried out for all studies, to make sure they are ethical, feasible, well-planned, have consulted with the necessary groups including patient groups, and to make sure the staff involved are suitable:
-
Health Research Authority/Health and Care Research Wales (HRA/HCRW) approval – the HRA issues a letter confirming that the study has been accepted from a governance point of view. Make sure that you see a copy of the approval letter, not just the initial assessment letter.
-
Research Ethics Committee (REC) approval – the HRA letter will refer to the REC approval letter where applicable. This confirms that ethics checks have been carried out. Ethical approval is not needed for staff-only studies or for some other types of study. If you are not sure please contact us: research@bradford.nhs.uk.
-
Organisation information document and Schedule of events – for non-commercial studies this explains what exactly will take place at your practice (if you agree), financial considerations and data arrangements. Practices must sign the organisation information document to agree that they have assessed and arranged their capability and capacity and are ready to proceed. For commercial studies an agreement will be in place which practices should sign to agree to take part. For studies where you are only being asked to put up a poster, there is no need for these documents, but you should ensure that the study is HRA approved before advertising the study.
-
Excess Treatment Cost (ETC) agreement – if the study treatment costs more than standard NHS care. If you are not sure whether an Excess Treatment Cost is involved please contact us: research@bradford.nhs.uk.
-
Advisory email regarding Capability and Capacity - the West Yorkshire R&D Team has an overview of research taking place in primary care and will review all studies and retain a copy of the document set. We will be able to advise your practice on whether there are any issues regarding capability and capacity, any overlaps with other studies, any over-use of a particular patient population. The study team should share this email with participating practices.
-
Letter of Access – if anyone is coming into your practice who is not a member of your practice staff, even if they are an NHS employee, they will need to show you a Letter of Access. This is issued by the West Yorkshire R&D Team to confirm that the person is suitably trained and checked to carry out the planned research.
When you have seen these documents, your practice must decide whether to take part. Once you have considered the implications of carrying out the study, if you have any questions please do not hesitate to contact us for advice: research@bradford.nhs.uk. If you are happy to proceed you need to sign the organisation information document or site agreement.
Study teams will usually hold a Site Initiation Visit to ‘kick off’ the study at your practice. Practice staff should attend if they are conducting any elements of the research.
You should receive a site file which should be held at your site and must be updated any time there is a change to the study. For an idea of what might be included in the site file please see suggested file contents on the NIHR website.
The Principal Investigator is responsible for all research activity taking place at the site. If someone else is to carry out a particular duty, for example a healthcare assistant may take blood samples, this should be documented to say that the Principal Investigator delegates that duty to the healthcare assistant. A full delegation log listing out all delegated duties should be held in the site file and signed by the Principal Investigator and delegates. CVs for any staff undertaking research at the site (including practice staff who are involved) should also be held in the site file.
Copies of all the latest versions of study documents – e.g. consent forms, patient information sheets, protocol, should also be kept in the site file.
Consent forms should be held securely but separately from the site file.
At the Site Initiation Visit, you should be given a process for reporting adverse events or issues, and a contact in the research team for any queries.
After the study
Your practice should retain the site file and consent forms after the study for an agreed period of time (agreed with the research team).
For health professionals research has a really important role in supporting evidence-based decision making and treatments. We are gathering information on our website that has arisen from research studies which have taken place in our area. This is a developing resource which will grow over time. We also provide links to accessing published evidence and research findings, many of which you may have access to through your NHS organisation or academic institution.
How we can help
Repository
You can access our repository here. Through our repository you can also access information about how to find and understand evidence reports.
Events
You can find information about events here, including events where research findings are shared and discussed.
You can read about our most recent events below:
If you would like to be informed about future events please contact us.
Practice Protected Time
We regularly hold stands at commissioner-led practice protected time events. We use these events as opportunities to engage directly with primary care professionals in our area, sharing information about the work we do and local research activity.
If you see our team at one of these events then please do feel free to approach us with any questions you have about NHS research or to share and discuss your own experiences of taking part in research in the area. Your feedback will help us to ensure that we respond to your needs, providing information that you will find valuable and pertinent. We also hope to help to address any barriers that may be preventing you from becoming engaged with research.
There is a large quantity of evidence available online and in print. The sheer quantity can be overwhelming and it is important to filter down the evidence to a manageable amount and to the most valuable and reliable evidence.
A good research article should always provide enough information so that the reader could, if they chose, recreate the study. This means the method, sampling strategy and all other elements of the study should be clearly described. This information also allows you to identify whether the study was well-run, whether an appropriate sampling strategy was used, what the statistical power of the study was, whether the study had any bias or conflicts of interest.
There is considered to be a hierarchy of evidence in clinical trials which is important as you can recognise whether the study type is of a high standard.
Health Knowledge describes the hierarchy of evidence, with randomised controlled trials being the ‘gold standard’ of a research study as a randomly selected group is studied with groups being randomised to different treatments. Control groups are used to evidence that any change is not due to other external factors. Any biases or confounding factors should be highlighted and controlled for where possible.
Above the randomised controlled trial in the hierarchy is a meta-analysis or systematic review which involves reviewing and, where possible, compiling, the results of a number of randomised controlled trials and sometimes other types of study. This highlights trends across multiple studies and can also highlight any publication bias, where some negative studies may not have been published.
As mentioned in the above article, this does not mean that other types of study are never useful as they can be. The hierarchy of evidence also does not incorporate qualitative research, as this methodology does not usually attempt to be generalizable or randomised, so cannot be approached in the same way. However qualitative research has a place in research - see this Nursing Times article for a discussion of the value of qualitative research.
Bear in mind that, although the label ‘randomised controlled trial’ indicates a high quality methodology, the detail of the study should be examined to make sure it was well-conducted and attempted to reduce bias.
Reading and understanding research findings, sometimes called ‘critical appraisal’, is a useful skill and can help you to recognise good research. Critical appraisal is useful for all types of research paper, including qualitative research.
Understanding health research talks you through doing a critical appraisal which can help you to identify whether a piece of research has been properly and rigorously carried out – in other words; is it worth reading, and trying to apply the results to your context? The tool walks you through the paper with a series of questions to help you to review the paper’s quality, and is very straightforward to use with hints, explanations and things to think about at each stage. You can also stop at any point and get a summary.
For training please see the following links:
The Critical Appraisal Skills Programme (CASP) also provides useful resources (tools and checklists) for reading and appraising research papers.
You may also like to read Trisha Greenhalgh’s book How to read a paper which is available in many libraries.
As we are all short of time, it can be useful to use another source which has already done the critical appraisal for us. This is why we recommend the Cochrane Library of systematic reviews and also NICE guidance. Watch a video on systematic reviews here.There is also information on NICE about how they put together the guidelines. There is a useful site the National Elf Service which produces summaries of evidence in a number of subject areas. However, it is useful to have the ability to appraise a research paper as you may be presented with a piece of research and asked for a response.
Glossary
A glossary of some of the key terms used in research is available from the National Institute of Health Research.
Evidence sources and library services
Cochrane Library of systematic reviews
National Elf Service – critically appraised expert commentaries
Bradford District Care Trust Library
Mid Yorkshire Hospitals NHS Trust Library and Information Service
Calderdale and Huddersfield NHS Foundation Trust Library Services
Critical appraisal
Leeds Libraries for Health Critical Appraisal Training
Critical Appraisal Skills Programme (CASP)
How to read a scientific paper
BBC Radio 4’s More or Less looks critically at statistics reported in the news.
This online community (WeCATS – Critical Appraisal Twitter Session) regularly critically appraises research papers via a Twitter chat.
Guides
Research methodologies
Statistical analysis and research methodology
Conducting research
Trial methodology – other training
Short courses in clinical research
Children and clinical research
Useful books
Understanding Clinical Papers Paperback – 8 Nov 2013 by David Bowers, Allan House, David Owens, Bridgette Bewick
Getting Started in Health Research - 10 Jun 2011 by David Bowers, Allan House, David Owens
How to Read a Paper: The Basics of Evidence-Based Medicine - 28 Mar 2014 by Trisha Greenhalgh
- Research In General Practice: Why Getting Involved in Research Might Be Easier—and More Rewarding—Than You Think - 21st July 2025. Written by Mathew Duke
- WAAW 2022: Antimicrobial stewardship and Lowering AntiMicrobial Prescribing (LAMP) - 18th November 2022. Written by Tasneem Khan.
- WAAW Day 7: Lowering AntiMicrobial Prescribing (LAMP) - 24th November 2021. Written by Tasneem Khan.
- WAAW Day 6: Taking AMR seriously in Leeds - 23rd November 2021. Visit Seriously Resistant.
- WAAW Day 5: Antibiotics and the Environment: What's the Link? - 22nd November 2021. Written by Costas Vasiliou.
- WAAW Day 4: Prescribing of antibiotics in Calderdale and West Yorkshire throughout the pandemic and issues surrounding remote prescribing of empirical antibiotics - 21st November 2021. Written by Nicola Booth.
- WAAW Day 3: A Patient's Story - 20th November 2021. Written by John.
- WAAW Day 2: The Origins and Resistance of Antimicrobials: A Community Pharmacist’s Advice to AMR - 19th November 2021. Written Robina Rowley-Conwy and Farial Majid.
- WAAW Day 1: World Antimicrobial Awareness Week & Lowering AntiMicrobial Prescribing (LAMP) - 18th November 2021. Written by Paul Carder.
- COVID-19: One Year On - 31st March 2021. Written by Stella Johnson.
- The Health Inequalities Academy - 10th February 2021. Written by Rose Dewey.
- LAMP in lockdown - the digital transformation - 30th June 2020. Written by Imran Mohammed.
- Increasing our primary care research activity – a practice manager’s perspective - 24th June 2020. Written by Nick Gwatkin.
- Research in COVID-19 - 15th April 2020. Written by Rose Dewey.
- Primary Care Networks: an emerging opportunity for research in general practice? - 29th January 2020. Written by Damian Reynolds.
- Lowering Anti-Microbial Prescribing (LAMP) - 19th November 2019. Written by Paul Carder.
- The 4th Annual International Symposium on Advancing the Science and Impact of Audit & Feedback - 30th July 2019. Written by Paul Carder.
- Thoughts following 2019’s R&D Forum - 31st May 2019. Written by Rose Dewey.
- A PhD Researcher’s Experience of WY R&D - 25th February 2019. Written by Kathryn Chater.
- A week in the (work) life! - 21st August 2018. Written by Rose Dewey.
- Research Delivery Officer Introduction - 30th July 2018. Written by Damian Reynolds.
- Research Co-ordinator Introduction - 20th July 2018. Written by Imran Mohammed.
- Reflecting on this year’s R&D Forum - 4th June 2018. Written by Rebecca Harper.
- Research in General Practice - 22nd March 2018 - Written by Emma Neil.
- A look back at the history of medical research - 12th February 2018 - Written by Emma Neil.
- Research Governance Lead Introduction - 30th January 2018 - Written by Rebecca Harper.
- Research Capability Funding Case Study - 31st August 2017. Written by Dr Matt Mulvey.
This guide offers practical, evidence-based advice on how to implement evidence-based care in general practice. You can download a full PDF version of this guide, or you can access individual sections below.

You can download a full version of this guide, or you can access individual sections below.
This guide is based upon a major research programme, Action to Support Practices Implementing Research Evidence (ASPIRE). The research was led by the University of Leeds and brought together collaborators including the West Yorkshire clinical commissioning groups, patients and the public, and representatives from the National Institute for Health and Care Excellent (NICE). Over 200 general practices from West Yorkshire took part in the research programme.





